NASA’s new Artemis program has humans returning to the lunar surface to establish a permanent sustainable presence. In order to accomplish this goal, new technologies will need to be developed to utilize the resources available on the Moon. Since oxygen is among the most critical elements for human survival, and one of the largest sources of launch mass, any technology able to utilize oxygen already on the Moon would be a critical enabler.
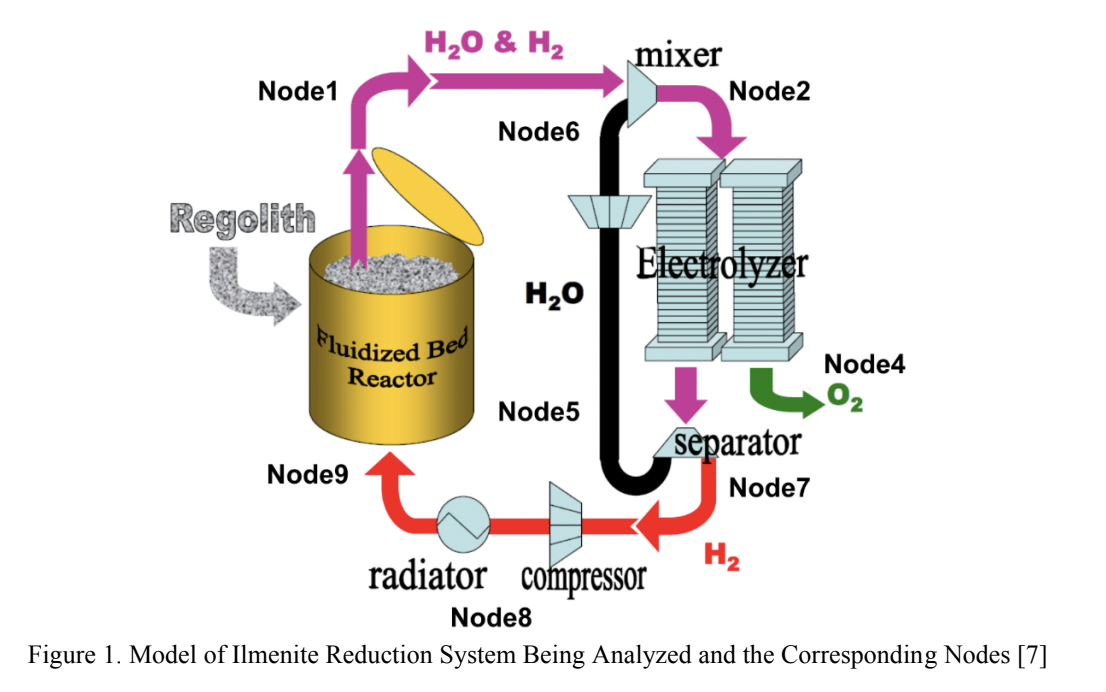
This paper discusses one possible In-situ Resource Utilization (ISRU) method for extracting oxygen from lunar regolith. The overarching process consists of two fundamental steps, first the reduction of regolith oxides with hydrogen gas, and second the electrolysis of water. The entire cycle from regolith to oxygen is described and a computational analysis is performed to determine first order approximations for oxygen production from ilmenite found in lunar regolith.
For detailed document, click here.
This study focused on deriving equations to represent the reaction, and accurately project the output percentages, rates, and masses. Many of the equations derived in that study were utilised in the study performed throughout this paper. A comparison was made between the study described in this paper to the results of both the computational and physical models described by predated studies. The purpose of the investigation was to analyse the feasibility of a chemical process plant, utilising ilmenite, to produce 15 metric tons of oxygen per year. With previous studies providing the proof of concept, the purpose was to take the ilmenite reduction process to the next step and develop a model that could inform NASA of needed materials. Although ilmenite can be readily found on the lunar surface, H2, may not be so abundant that enough exists and can be captured into the reactor to sustain a reaction for a year. This model aimed to be able to set standards for input feedstock to the chemical reactor, as well as be able to provide a required amount of hydrogen needed to feed the reactor for any given length of time. This would enable the customer to calculate how much hydrogen would need to be provided on a resupply mission for a given amount of time on the Moon. It was hypothesised that an increase in lunar regolith as well as an increase in hydrogen provided to the system would result in the direct increase of oxygen production, and that no critical mass would be reached to cause adverse effects to the reaction output.
For detailed document, click here.
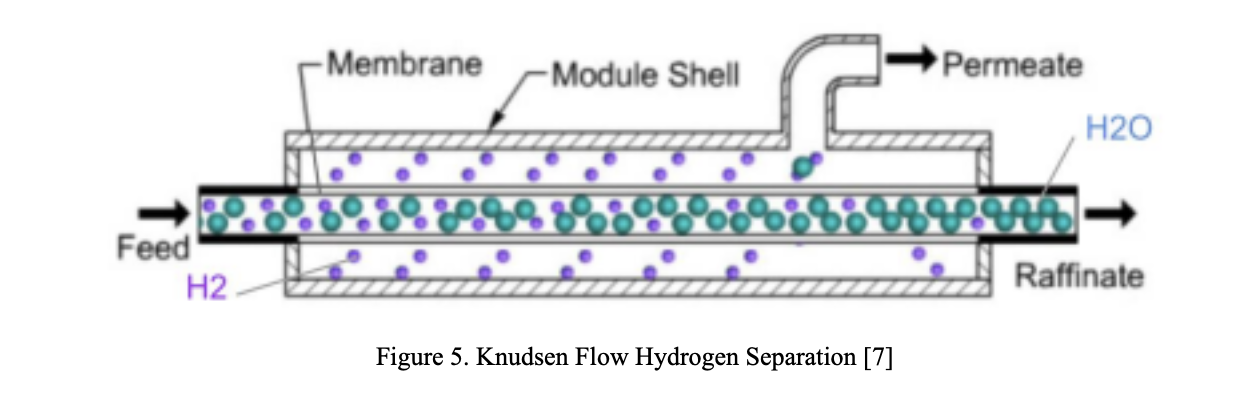
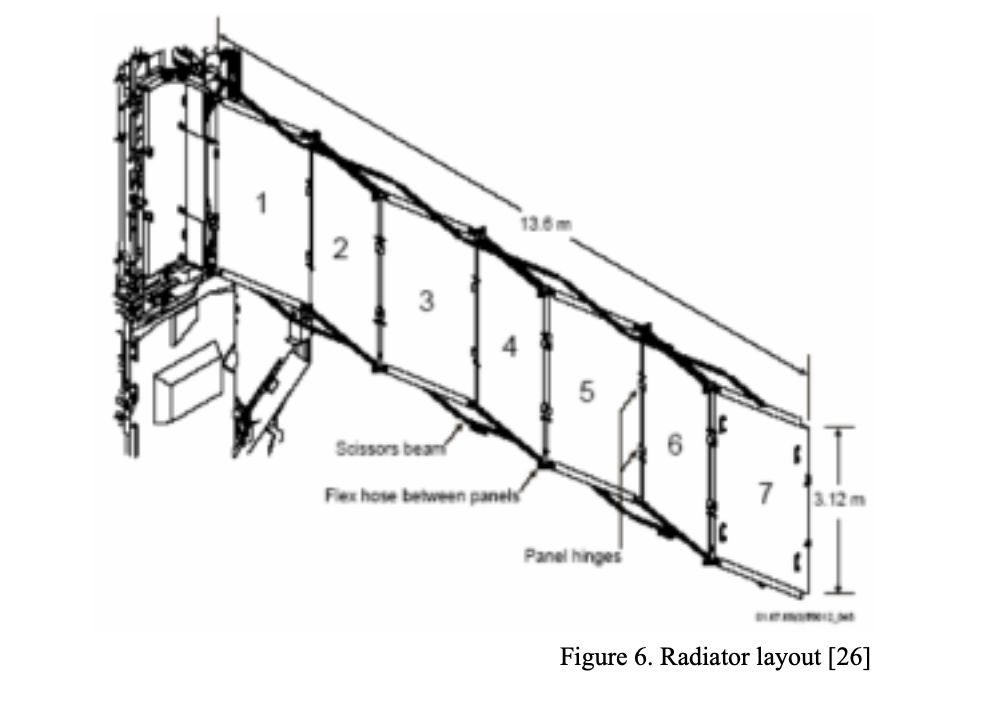
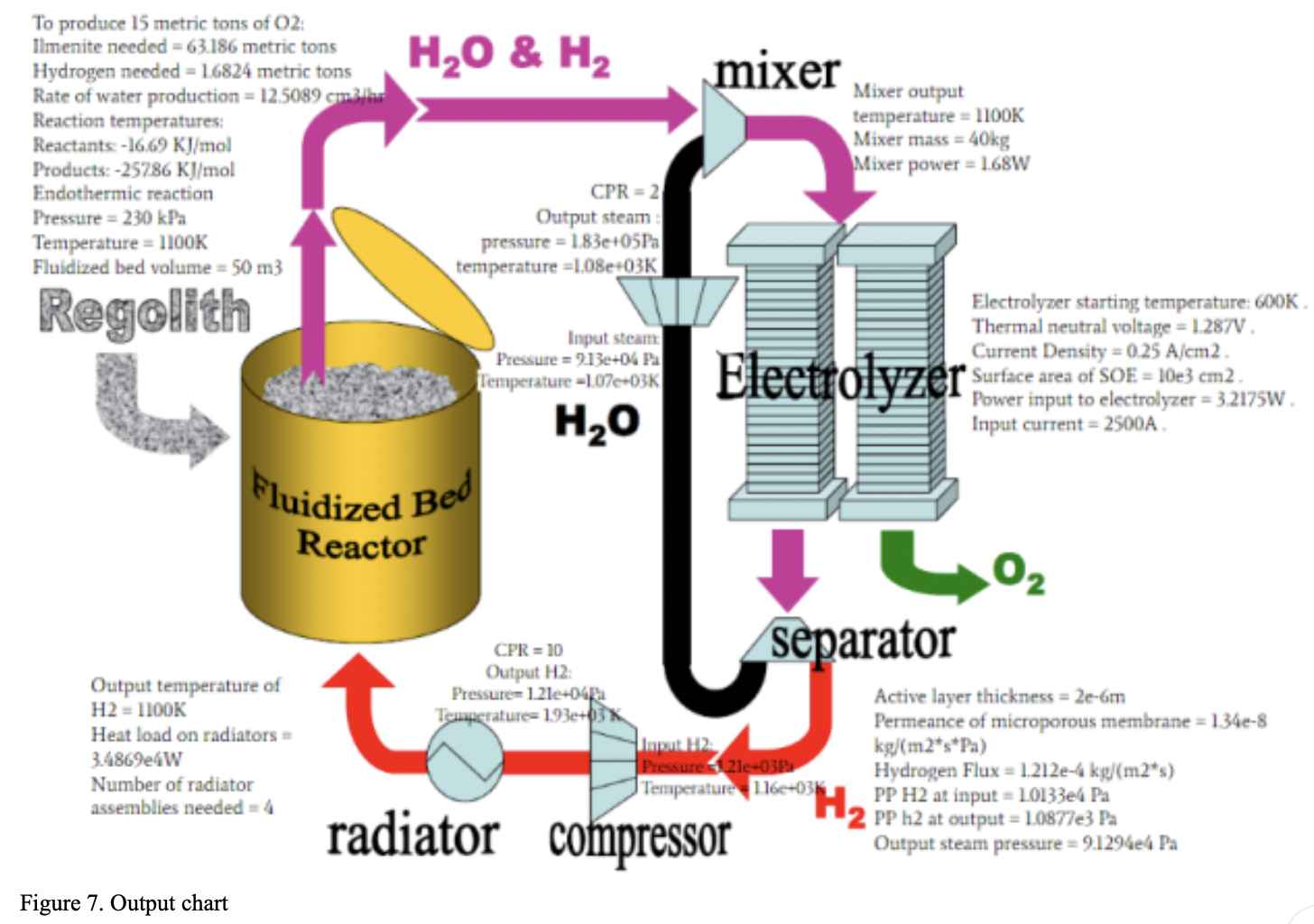
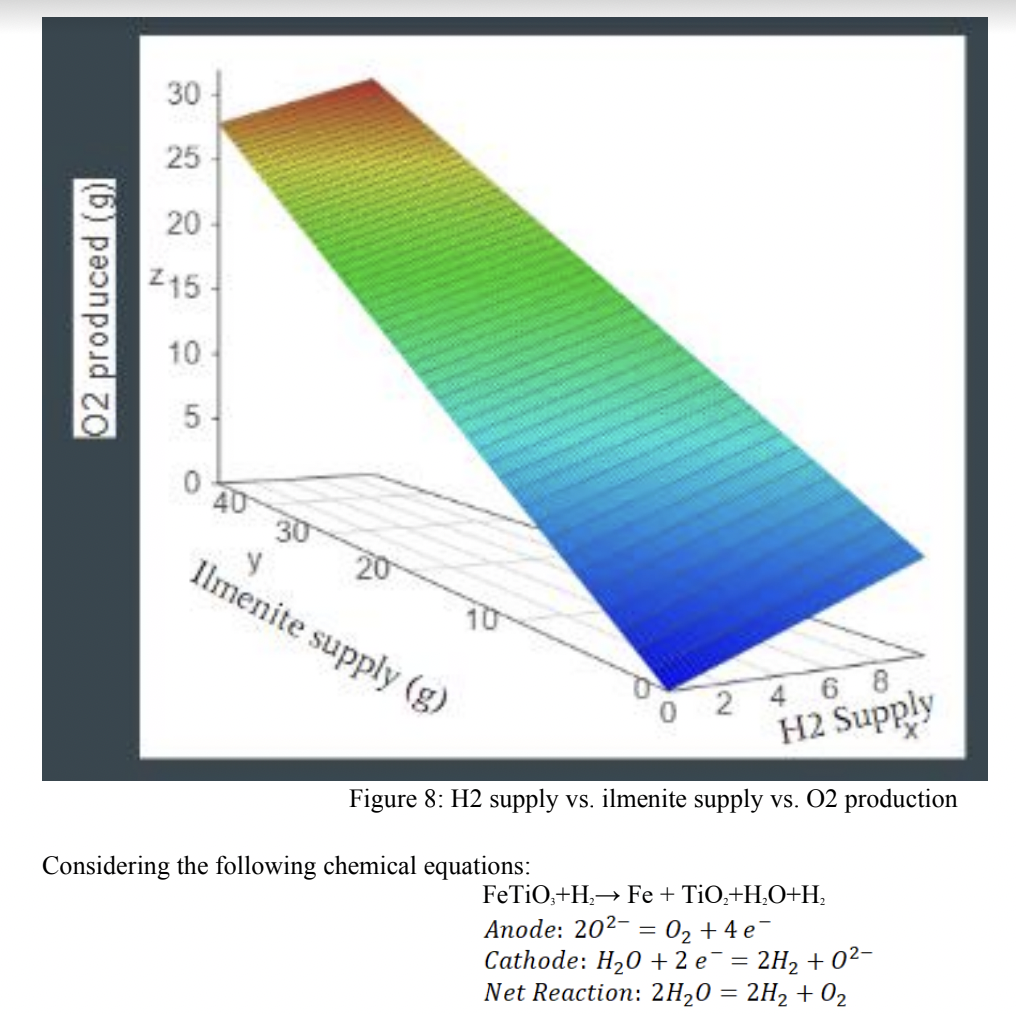
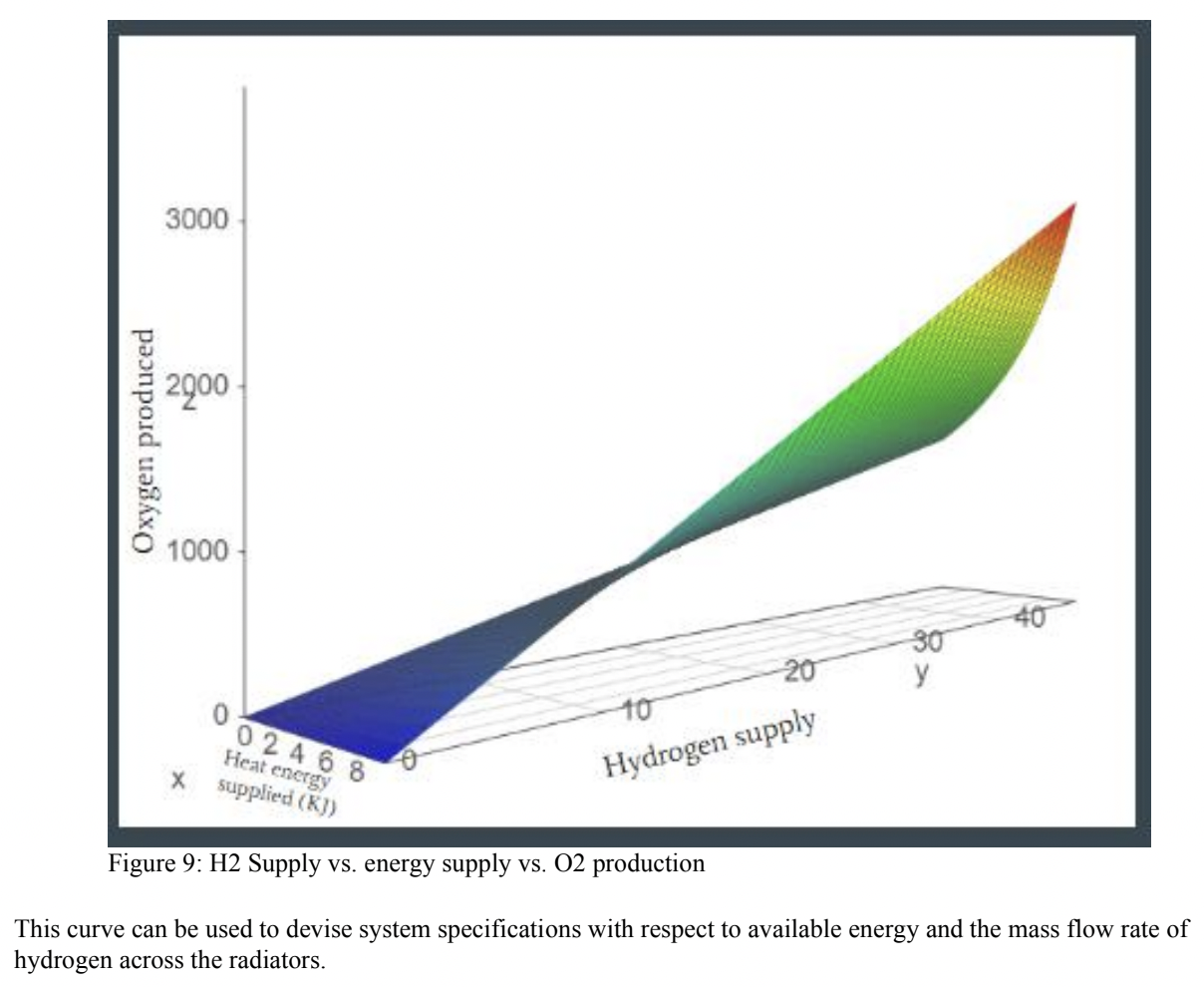
Results:
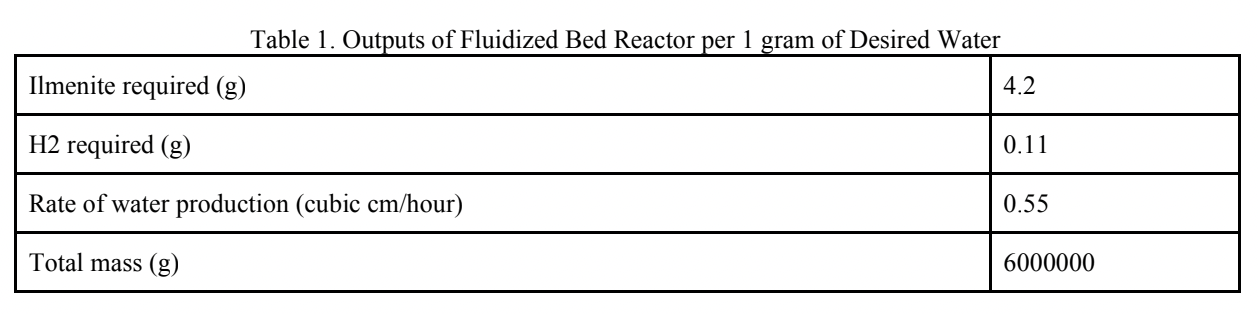
A computation trial was performed to calculate system inputs and outputs for 15 metric tons of oxygen production and the results were summarised as:
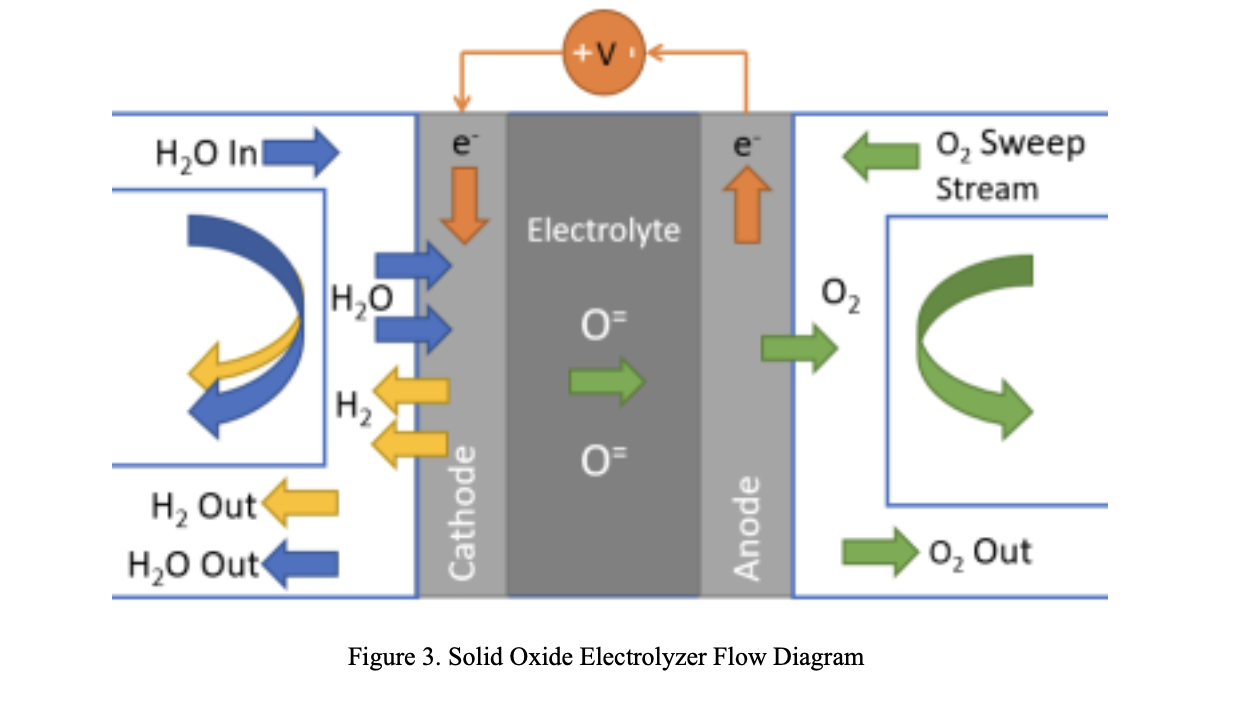
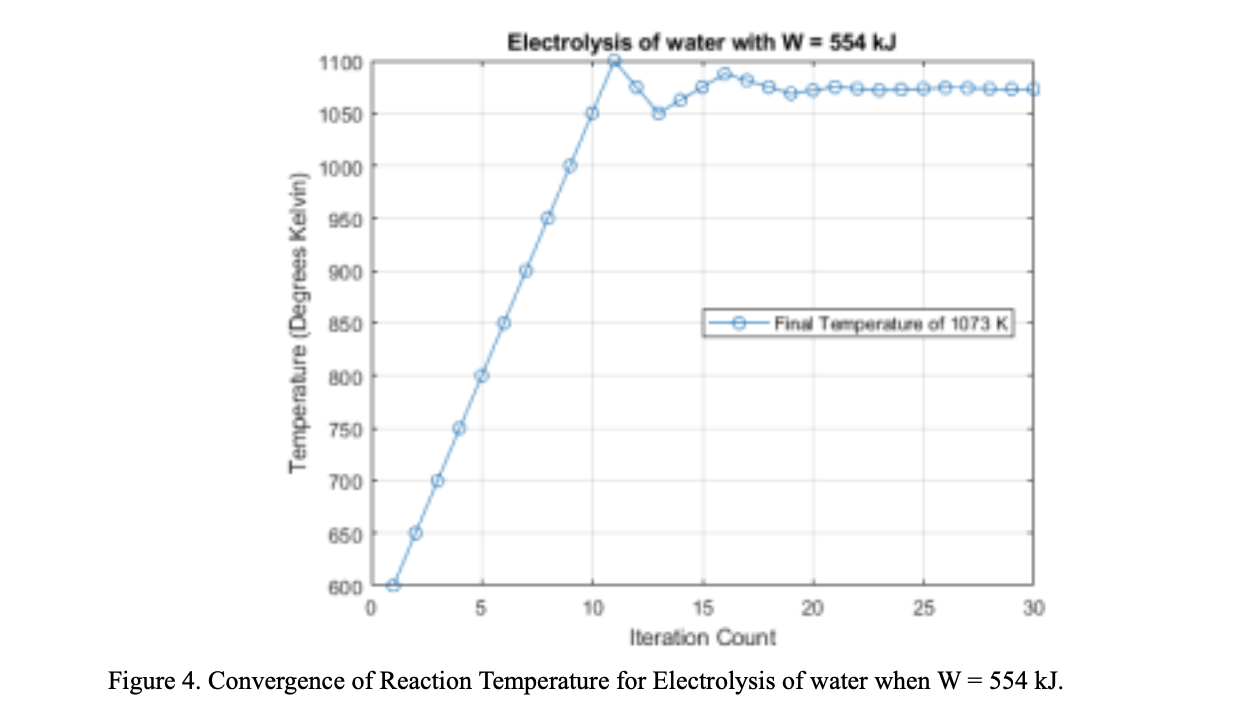
To produce the 15 metric tons of oxygen, 63.18 metric tons of ilmenite would need to be input into the reactor along with 1.68 metric tons of hydrogen. The pressure and temperature of the fluidized bed reactor would stay constant at 1100K and 230 kPa, respectively. With these metrics the rate of water production which feeds the mixer would be 12.5 grams per hour. Feeding the resulting water from the fluidized bed reactor to the mixer using these masses would result in 15 tons of oxygen at the end of the system process. In this analysis, it was assumed that there is no chemical change or pressure drop in the mixer. Thus, the variable is the only temperature, and it is dependent on the input from the fluidized bed reactor and H2O compressor, and it is in the range of 1100 K. At this rate 12.5 grams of water/hour gets split by the SOE. The SOE Generates 2 mols of hydrogen gas and one mol of oxygen gas for every 2 moles of steam. Therefore, at a rate of 12.5 grams of water per hour, the SOE generates 1.3g of Hydrogen gas /hour and 11.11g of Oxygen per hour. In the separator, pressure drops are 100 kPa and 10 kPa in permeate side and raffinate side respectively. These pressure drops are compensated by different small compressors. The steam compressor outputs are in the range of 183 kPa and 1080K. This steam is sent to the mixer to be re-sent through the electrolyzer. The hydrogen compressor outputs are in the range of 12kPa and 1930K. This pressurized hydrogen gas is then passed through the radiator to be cooled to 1100K. A hydrogen mass flow rate of 0.5m^3/s would demand 4 PVR assemblies
References:
[1] Brown, L., Peick, J., Pickett, M., Fanara, T., Gilchrist, S., Smiley, A., & Roberson, L. (2021). Aquatic invertebrate
protein sources for long-duration space travel. Life Sciences in Space Research, 1-10.
[2] Carr, Bruce B.. "Recovery of Water or Oxygen by Reduction of Lunar Rock". AIAA Journal, vol 1, no. 4, 1963,
pp. 921-924. American Institute Of Aeronautics And Astronautics (AIAA), doi:10.2514/3.1674.
[3] Taylor, L.A., Carrier, W.D., 1993. Oxygen production on the Moon: an overview and evaluation. In: Lewis, J.,
Matthews, M.S., Guerrieri, M.L. (Eds.), Resources of Near-Earth Space. University of Arizona Press, Tucson, AZ,
pp. 69–108.
[4] "NASA: Artemis". NASA, 2021, https://www.nasa.gov/specials/artemis/. Accessed 28 Apr 2021.
[5] Simon, Tom, and Kurt Sacksteder. "NASA In-Situ Resource Utilization (ISRU) Development & Incorporation
Plans". 2007.
[6] Sargeant, H.M. et al. "Hydrogen Reduction Of Ilmenite: Towards An In Situ Resource Utilization Demonstration
On The Surface Of The Moon". Planetary And Space Science, vol 180, 2020, p. 104751. Elsevier BV,
doi:10.1016/j.pss.2019.104751.
[7] C. J. Steffen, Jr., J. E. Freeh, D. L. Linne, E. W. Faykus, C. A. Gallo, and R. D. Green, 2007. System Modeling of
Lunar Oxygen Production: Mass and Power Requirements. Proceedings of Space Nuclear Conference Paper 2049
[8] Altenberg, B et al. "Thermodynamics Of Lunar Ilmenite Reduction". NASA Astrophysics Data System, 1993, pp.
1-2., http://adsabs.harvard.edu/pdf/1993LPI....24...27A. Accessed 28 Apr 2021.
[9] J.N. Rasera, J.J. Cilliers, J.A. Lamamy, K. Hadler, 2020. The beneficiation of lunar regolith for space resource
utilisation: A review. Planetary and Space Science v.186, pp 1-15.